|
Clinical Characteristics and
Inheritance of Idiopathic Epilepsy
Edward (Ned) Patterson, DVM, PhD, DACVIM (SAIM)
University of Minnesota College of Veterinary Medicine, St. Paul, MN, USA
Objectives of the Presentation
Overview of the Issue Idiopathic Epilepsy is repeated seizures over time in which an underlying cause is not determined. Typically dogs affected with IE have their first seizure from 6 months to 6 years of age. Most IE is considered to have a genetic basis and is a difficult for breeders to select against because of the late onset of the disease and the lack of identification of carriers. The lifetime incidence of Idiopathic Epilepsy (IE) in dogs is reported to be from 0.5% to 5% depending on the breed (Podell 1995). Often an affected dog has already been bred before the condition is diagnosed. Inherited forms of IE are currently known to exist in many different dog breeds although to date the genetic bases have not yet been determined. For the breeds in which there have been scientific studies of the mode of inheritance, it has been suggested to be either simple recessive or polygenic recessive inheritance. Under both types of inheritance canine epilepsy is a difficult trait to control based on phenotype alone, since it can be also be passed on by carrier animals that are not themselves clinically affected. Summary Many inherited diseases in breeds of dogs are caused by a "founder" effect, which is also the basis of many genetic diseases found in isolated human populations. Inherited or idiopathic epilepsy (IE) is a common canine disease causing repeated seizures and is the most common neurological disease of dog and is the one of the top 3 overall health concern for many dog breeds (AKC Survey 2001). IE must be differentiated from secondary epilepsy in which there is an underlying cause such as brain tumor, brain malformation, brain infection, low blood sugar, low blood calcium, or liver failure. A search of idiopathic epilepsy diagnoses from all contributing North American Veterinary Colleges was performed by comparing breed, age, and sex of all first time canine admissions for epilepsy in a specific breed versus all admissions for that specific breed from 1987 to 1997. Overall, the percentage of epilepsy admissions by breed ranged from 0.12% to 2.01% with an all-breed average, including mixed breeds, of 0.82% (Table 1). Table 1 - Percentage of First Times Admissions by Breed for Canine Idiopathic Epilepsy
Peer-reviewed scientific studies indicating a genetic basis for idiopathic epilepsy are available for Beagles (Bielfelt 1971), German Shepherds (Falco 1974), Keeshonds (Hall 1996), Dachshunds (Oliver 1994), Golden Retrievers (Srenk 1994), Labrador Retrievers (Leinweiner 1999), Bernese Mountain Dogs (Kathmann 1999), Vizslas (Patterson 2003), Belgian Tervurens (Oberbauer 2003), and English Springer Spaniels (Patterson 2005). These publications have indicated possible modes of inheritance of IE, but the molecular bases are unknown at this time. A brief summary of the variety of known seizure characteristics and suggested modes of inheritance of canine epilepsy is presented in Table 2. Table 2.
It is very likely that many more breeds have heritable forms of IE but insufficient data has yet been collected to characterize it. The multiple modes of inheritance identified in the studies to date, as well as the diverse nature of the breeds involved, make it highly likely that there will be many different genetic causes for canine epilepsy. It is possible, though, that related breeds with similar IE phenotypes may have a similar genetic basis. The only anti-epileptic drugs that are consistently effective in dogs are phenobarbital and bromide (Podell 1996). Euthanasia may be chosen due to frequency or severity of seizures in cases in which these drugs are not effective. There is some controversy in the veterinary community regarding partial seizures in dogs and the cause of the seizure disorder (idiopathic versus secondary). By definition, a partial seizure originates in a small region of the brain. A generalized (grand-mal type) originates simultaneously in most of the outer parts of the brain. Some authors believe that partial seizures are likely to indicate an underlying structural brain abnormality (Berendt 1999, Knowles 1998), whereas others believe that partial seizures can have a heritable basis with no underlying brain abnormality and therefore be considered IE (Thomas 2000, Podell 1996). A recent study classified 65% of epileptic dogs as having partial onset seizures and 32% as having primary generalized (grand-mal type) seizures (Berendt 1999). In our analysis of seizures in Vizslas (Patterson 2003) seventy-nine percent of the dogs affected with IE had partial seizures with or without secondary generalization. Partial seizure signs consisted of a combination of leg tremors, staring, dilated pupils, salivation, or some combination of these without loss of consciousness in over 79% of the affected individuals. Thus, there is increasing evidence that partial seizures, in which the seizures start in the age range of 6 months to 6 years of age, are often heritable in dogs and therefore can be classified as IE. Epilepsy also affects approximately 1% of the human population, and the causative mutations in 22 very rare forms of human IE have been recently reported (Gurnett 2007). However, the most common idiopathic human epilepsies have complex (polygenic) inheritance (Berkovic 1999). Since 15 of the 22 genes currently identified as causative of human IE are ion channels sodium (Na+), potassium (K+), calcium (Ca++), and chloride (Cl-) - (Gurnett 2007), it is suspected that ion channel defects may be involved in the more common polygenic forms of IE in people (Steinlan 1998), and could possibly be involved in canine epilepsy. However, there are hundreds of different potassium, chloride, sodium, and calcium channels, often with multiple sub-units, expressed in the brain(Lehmann-Horn 1999). Given the complex mechanisms regulating excitability in the brain it is likely that hundreds of genes could potentially cause IE. There are a number of ongoing projects trying to determine the gene or genes that cause IE in various dog breeds at the University of Minnesota, the University of Missouri -Columbia, The University of California - Davis, The University of Toronto, The Animal Health Trust in England, A University in Finland, and at a few other institutions.
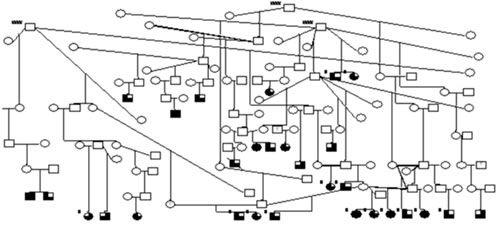
In our lab, we have made the most progress in Vizslas. To date, we have collected 86 affected Vizslas and 606 related family members. In 2003, we published an initial analysis of 29 affected Vizslas, indicating that most Vizslas have partial onset seizures and that the mode of inheritance is consistent with autosomal recessive (table 3), but that polygenic inheritance is also possible (Patterson 2003). We have checked 448 DNA markers over all 39 canine chromosomes for our entire collection of Vizsla families. We have excluded approximately 92% of the genome from containing an epilepsy gene based on the possible simple recessive inheritance. We currently have found 3 markers with suggestive but not conclusive linkage (association) to IE in Vizslas on one specific canine chromosome. We have also used another strategy for finding genes for IE in Vizslas. As mentioned, Ion channels (Na+, CA++, K+, Cl- etc) have been found to cause epilepsy in 15 of 22 mutations defined in isolated human populations. We have now identified 50 unpublished microsatellite markers near Ca++ channels, Na+ channels, K+ channels, GABA and Acetycholine receptor ion channels and have checked our Vizsla family collection with them. In the past 14 months, genetic analysis of 18 Ca++ channels, 12 K+ channels, and 8 NA+ channels have excluded them as being a causative gene for IE in these Vizslas families. We are continuing to test the remaining genes at this time. In addition we have tested a number of affected Vizslas for the mutation causing progressive myoclonic epilepsy (Lafora Disease) in Dachshunds (Lohi et al. 2005) and none of these affected Vizslas have this particular mutation. In summary, to date we have tested 486 genetic markers on our Vizsla samples. We have identified a possible area for a Vizsla IE gene in a small span of canine one specific canine chromosome. This Chromosome span is likely to contain a gene contributing to Epilepsy in Vizslas and we are studying additional markers in the area. We are concerned, however, that IE in Vizslas could be controlled by two or more genes that would be unlikely to be detected with our current methods. We plan on next using thousands of single nucleotide polymorphism (SNP) based genetic markers that have just been developed. These SNP markers have the potential to detect chromosomal areas for complex genetic traits in which 2 or more genes cause a disease. Since the most common mode of inheritance for IE in dogs is either simple autosomal recessive or polygenic recessive it will be very difficult to eliminate carriers from breeding until conclusive genetic tests are developed. Given emerging DNA technology for dogs, in a few dog breeds genetic mutations are likely to be identified in the next 3-5 years. Given, that different breeds (unless closely related) are likely to have different genes involved, it may be many more years until conclusive genetic tests are developed in some other breeds. If a breeder is having difficulty which IE in their lines, the best that can be done for now is to have a canine geneticist do carrier risk analysis for those breeds in which IE has been suggested to be simple autosomal recessive (Table 2). For breeds in which IE has been suggested to be polygenic or breeds in which the inheritance is unknown then depth and breadth of pedigree analysis should be done (as is done for other polygenic traits such as hip dysplasia) with the recommendation that no ancestors in the depth of pedigree are affected with IE, and that <25% of siblings are affected with IE in the breadth of pedigree analysis. In addition for lines with an IE problem it is suggested that one or both of the breeding individuals also be at least 5 years of age in order for it to be likely that they are not affected with IE. Once conclusive genetic tests are developed for a breed then breeding recommendations will be much more concrete, and more likely to rapidly decrease the incidence of IE. To help these studies progress we encourage individuals to submit DNA of purebred dogs affected with IE and their close relatives to the various studies. In the case where more than one group is studying the same breed, I recommend individuals submit samples to all groups performing the studies. References/Suggested Reading 1. Berendt M, Gram L (1999). Epilepsy and seizure classification in 63 dogs: a reappraisal of veterinary epilepsy terminology. J Vet Intern Med 13:14-20. 2. Berkovic, S. F., and Scheffer, I. E. (1999). Genetics of the epilepsies. [Review] [53 refs]. Current Opin in Neur 12, 177-82. 3. Bielfelt SW, Redman HC, McClellan RO. (1971). Sire- and sex-related differences in rates of epileptiform seizures in a purebred beagle dog colony. Am J Vet Res. 32: 2039-2048. 4. Falco, M.J., Barker J., and Wallace, M.E.(1974) The genetics of epilepsy in the British Alsatian. J. Small Anim. Pract. 15, 685-692. 5. Gurnett PA, Herera P (2007) New ideas in epilepsy genetics: novel epilepsy genes, copy number alterations, and gene regulation. Archives of neurology (64):324-8. 6. Hall SJ, and Wallace ME (1996) Canine epilepsy: a genetic counseling programme for keeshonds. Vet Rec 138(15): 538-60. 7. Hartl DL, Jones EW (1998). Genetics: principles and analysis. 4th ed. Sudbury MA: Jones and Bartlett Publishers,; 109-114. 8. Kathmann, I., Jaggy, A., Busato, A., Bartschi M., and Gaillard, C. (1999) Clinical and genetic investigations of idiopathic epilepsy in the Bernese mountain dog. J Small Anim. Pract 40, 39-325. 9. Knowles K. Idiopathic epilepsy. Clin Tech Small Anim Pract 1998;13(3):144-151. 10. Lander, E. & Kruglyak, L (1995). Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11, 241-7 11. Lehmann-Horn, F., and Jurkat-Rott, K. (1999) Voltage-gated ion channels and hereditary disease. Physio. Rev. 79, 1317-1372. 12. Leinweiler C. Jaggy A (1999). Clinical, epidemiologic and therapeutic aspects of idiopathic epilepsy in 25 golden retrievers: results of a long term study. Schweizer Archiv fur Tierheilkunde. 141(5):231-8. 13. Lindblad -Toh, K, Wade, CW, Mikkelsen TS, Karlson EK, et al. (2005). Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438: 803-19. 14. Lohi H, Young EJ, Fitzmaurice SN, et al. (2005). Expanded repeat in canine epilepsy. Science 307: 81. 15. Mulley JC, Scheffer IE, Petrou S, and Berkovic SF (2003). Channelopathies as a genetic cause of epilepsy. Curr Opin Neurol 16:171-176. 16. Oberbauer AM, Grossman DI, Irion DN, Schaffer AL, Eggleston ML, and Famula TR (2003). The genetics of epilepsy in the Belgian Tervuren and Sheepdog. J. of Hered. 94(1):57-63. 17. Oliver, J.E. and Lorenz, M.D. (1993) Handbook of Veterinary Neurology. WB Saunders Co., Philadelphia. 18. Ostrander EA, Kruglyak L. (2000). Unleashing the canine genome. Genome Res. 10: 1271-1274. 19. Patterson EE, Da Y, Mickelson JR, Roberts MC, McVey A, O'Brien D, Johnson GS, Armstrong PJ. (2003) Clinical characteristics and inheritance of idiopathic epilepsy in Vizslas. Journal of Veterinary Internal Medicine, 17(3) 319-325. 20. Patterson EE, Armstrong PJ, Roberts MC, O'Brien D, Johnson GS, Mickelson JR. Clinical Description and Inheritance of Idiopathic Epilepsy in English Springer Spaniels. Journal of the American Veterinary Medical Association, 226(1): 54-59. 21. Podell, M. (1995) Seizure classification in dogs from non-referral based population. J Am Vet Med Ass 6:1721-8. 22. Podell, M. Seizures in dogs (1996). [Review] [84 refs]. Vet Clin N Am Sm An Pract 26, 779-809. 23. Sander, T. et al. (2000). Genome search for susceptibility loci of common idiopathic generalized epilepsies. Hum Mol Genet 9, 1465-72. 24. Srenk, P., Jaggy, A., Gaillard, C., Busato, A., Horin, P. (1994). Genetic aspect of idiopathic epilepsy in the Golden Retriever. Tierarztl Prax 22:574-578 25. Steinlein, O. K. (1998) New insights into the molecular and genetic mechanisms underlying idiopathic epilepsies. Clin. Genet. 54, 169-175. 26. Thomas WB. Idiopathic epilepsy in dogs. Vet Clin North Am: Sm Anim Pract2000;30(1):183-205.
Speaker Information
(click the speaker's name to view other papers and abstracts submitted by this speaker)
Edward (Ned) Patterson, DVM, PhD, DACVIM (SAIM) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||